News
Passion, dedication, innovation, enterprising, integrity, truth-seeking, altruism, win-win

Passion, dedication, innovation, enterprising, integrity, truth-seeking, altruism, win-win

You spend a large part of your budget on high-quality pipes, expecting them to last for decades. But then, only a year later, you see red rust spots appearing on the surface. This failure creates safety risks and ruins your project budget. Stainless steel corrosion resistance depends on a self-repairing "passive layer" of chromium oxide. When this layer remains intact, the metal stays rust-free. However, specific environments like strong acids, chlorides, or poor welding can break this shield, leading to structural failure.

We often assume that "stainless" means it will never stain. This is a dangerous misunderstanding in our industry. If we do not understand the specific environment, even the most expensive materials will fail. Let us look at the science to save your project money.
Selecting the wrong material grade for a chemical plant or a pipeline is a disaster waiting to happen. If the acid inside the pipe eats through the wall, production stops, and you face huge replacement costs. General corrosion happens when the entire surface dissolves uniformly. While grades like 304 handle nitric acid well, you need Grade 316 or 904 for sulfuric and phosphoric acids. Never use standard stainless steel with hydrochloric acid without expert consultation.

When we talk about general corrosion, we are talking about a uniform attack. The acid eats away the pipe wall at a steady speed. As a purchasing manager, you actually prefer this over other types because it is predictable. You can calculate the life of the pipe. However, you must choose the right grade. Acids are the biggest enemy here. In my experience at Centerway Steel supplying global oil and gas companies, I see many mistakes with Sulfuric Acid. Grades 304 and 316 can handle it, but only if the concentration is very low (under 10%) or very high. In the middle range, the corrosion is fast. For Phosphoric acid, usually found in fertilizer plants, Grade 316 is the standard choice. However, Hydrochloric acid is different. It will destroy the passive layer of most standard stainless steels very quickly. If your project involves this acid, we need to look at special alloys. Bases (alkalis) are usually safer. Stainless steel handles weak bases well. But, you must be careful with chlorides like Sodium Hypochlorite. High temperatures combined with strong bases can cause cracking. Organics generally do not hurt the stainless steel corrosion resistance of the 300-series (like 304 and 316). This is why these pipes are popular in food processing and oil storage. But always check the temperature. Heat makes every chemical reaction faster and more dangerous.
Uniform rust is easy to spot during an inspection, but what about the small holes you cannot see? These hidden traps cause sudden leaks and can shut down an entire construction project without warning. Localized attacks like pitting and crevice corrosion are dangerous because they are hard to detect. Pitting creates small holes due to chlorides, while crevice corrosion happens in tight gaps. Galvanic corrosion occurs when two different metals touch in moisture.
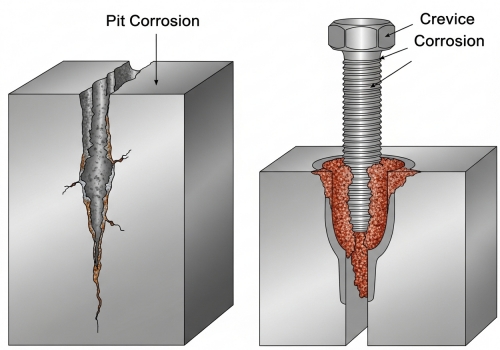
Unlike general corrosion, you cannot predict when a pipe will fail from localized corrosion. This is a nightmare for engineers and buyers. Pitting is one of the most destructive forms. It looks like a pinprick on the surface, but underneath, it creates a deep cavity. This usually happens when the environment has a lot of chlorides, like seawater or salt air. Since I work with many clients in coastal construction and offshore drilling, I always recommend Grade 316 or Duplex steels for these areas. These grades contain Molybdenum, which specifically fights pitting. Crevice Corrosion is a design issue. It happens in tight spaces where fluid doesn't move, like under a gasket, a bolt head, or under huge deposits of dirt. In these gaps, the oxygen runs out. Remember, stainless steel needs oxygen to repair its protective layer. Without oxygen, the layer breaks down, and the metal rots. Good design and regular cleaning prevent this. Galvanic Corrosion (or bimetallic corrosion) is a mistake I see in mixed systems. If you connect a carbon steel pipe directly to a stainless steel pipe, and there is water present, the carbon steel will rust much faster. The stainless steel acts as a cathode and "eats" the carbon steel. You must use insulating kits or gaskets to separate different metals. Here is a simple table to help you identify the risks:
| Corrosion Type | Primary Cause | Best Prevention Strategy |
|---|---|---|
| Pitting | Chlorides (Salt), low flow | Use Molybdenum grades (316, 2205). |
| Crevice | Stagnant fluid in gaps | Better joint design, avoid deposits. |
| Galvanic | Dissimilar metals touching | Insulate connections (nylon washers). |
| Galling | Friction between moving parts | Use different hardness levels for bolts/nuts. |
You buy the best material available, but the welder makes a mistake on site. Suddenly, the area right next to the weld starts to rust. This is a common quality control nightmare. Weld decay, or intergranular corrosion, happens when steel gets too hot (550°C-850°C). This sucks the chromium out of the grain boundaries. The solution is using Low Carbon grades like 304L or 316L to prevent this reaction. We must understand what happens during welding. To melt the metal, you apply high heat. If standard stainless steel stays in the temperature range of 550°C to 850°C for too long, the Carbon in the steel grabs the Chromium. They form "Chromium Carbides." Why is this bad? The stainless steel corrosion resistance comes from Chromium. If the Chromium is tied up with Carbon, it cannot form the protective oxide layer on the surface. The area near the weld becomes weak and rusts easily. This is called "Sensitization." For an EPC company, the fix is simple but important. When you order pipes that will be welded, you should specify "L" grades. 304L and 316L have very low carbon content (less than 0.03%). Because there is less carbon, it cannot steal the chromium, and the corrosion resistance stays high even after welding. Another method is using "Stabilized" grades like 321 or 347. These contain Titanium or Niobium. These elements love carbon more than chromium does. They grab the carbon first, leaving the chromium free to protect the pipe. At Centerway Steel, we produce large diameter and heavy thickness pipes. Welding these thick pipes takes a long time, which means more heat. Therefore, we always control our welding procedures strictly and often use post-weld heat treatment to reset the metal's structure. This ensures the pipe you receive is safe.
Stainless steel corrosion resistance relies on protecting the passive chromium layer. By choosing the correct grade (like 316L for acids or welding) and avoiding design traps, you ensure your project's longevity.